Better Infusion Pump choise for 5-FU
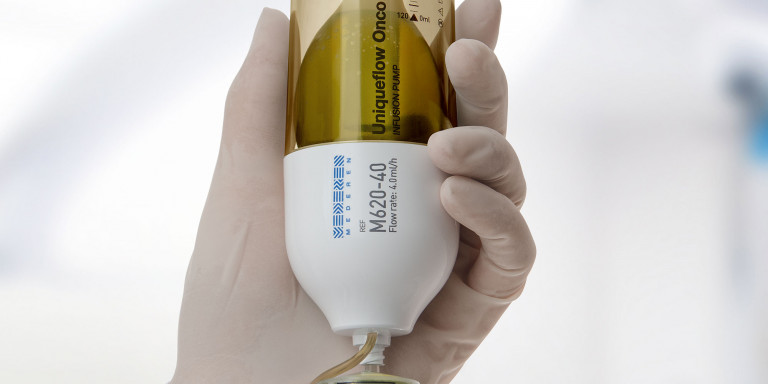
Because of its long infusion time over 46–48 hours, 5-fluorouracil (5-FU) is usually administered with an ambulatory infusion pump in the outpatient setting. However, two types of pumps exist. On one side of the spectrum is the elastomeric pump: small, compact, but gets the job done. On the other is the electronic pump: bigger, flashy, with lots of bells and whistles (literally). Here are the advantages and disadvantages of each.
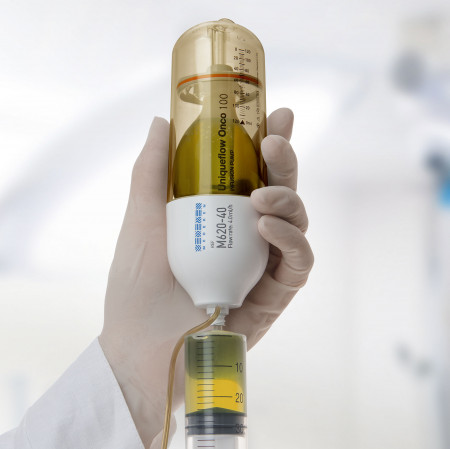
Elastomeric Infusion Pumps
At first glance, the elastomeric pump appears simple. Using a balloon inside a plastic covering, the unassuming device has the capability to infuse a variety of medications at different rates. It has no buttons to program it; rather, it relies on an elastomeric membrane to generate pressure that moves the fluid out of the membrane. The rate of infusion is controlled by an inline orifice or flow restrictor.
Studies have shown that patients and clinicians prefer elastomeric pumps over electronic. Clinicians cited benefits such as minimized risks related to pump programming, ease of patient teaching, reduced filling time in the pharmacy, and ease of patient use. Patients liked the lack of noise, lightweight design, and simplified use.
However, the lack of an alarm system can be a safety risk. Some clinicians argue that the elastomeric pump can fluctuate significantly in the accuracy of infusion time because of factors like temperature or viscosity: one study showed that 40% of elastomeric pumps had excess solution left at disconnect. And some insurances may not cover it because it is disposable.
Electronic Pumps
Electronic pumps require a power source and usually operate via peristaltic mechanisms that propel the infusion forward via appendages that move in waves (similar to peristalsis of the bowel). Rates are programmed into the pump as either intermittent or continuous infusions. Many come with audible and visual alarms to alert users to errors such as occlusion, low battery, and pump malfunction.
Although electric pumps are often seen as more dependable than elastomeric pumps because they must be programmed, the act of programming is also considered one of the risks because of the potential for user error. In addition, electronic pumps are often sensitive to radiation exposure and can result in pump malfunction for patients on concurrent therapy.
Safety Concerns
The case of an accidental fatal overdose of 5-FU delivered via an electronic pump led many to believe that it is a higher-risk pump. However, more recent reports of accidental 5-FU overdoses have involved both electronic and elastomeric pumps; illustrating neither are free of risk. More important factors for decision include patients’ ability to troubleshoot the pump, the pump’s weight and availability, and expense or insurance coverage.
To help address the patient and provider safety issues associated with ambulatory infusion pumps, the U.S. Food and Drug Administration developed the Infusion Pump Improvement Initiative in 2010. Safety recommendations from that program and the Institute for Safe Medication Practices follow.
- Ensure any staff who order, dispense, or administer 5-FU and other chemotherapy agents obtain and maintain skill and knowledge before working independently.
- Prescribe 5-FU clearly in single, daily doses with directions to infuse continuously over a certain number of days or hours.
- Standardize pharmacy labels to ensure that key information (e.g., name of medication or fluid, total volume, concentration, hourly rate) is displayed in a standard and consistent way, eliminating any unnecessary information.
- Enhance independent double checks (e.g., infusion volume, pump programming, pump settings) with a structured process for completing and documenting (e.g., checklists); establish a process for how this step is accomplished when the medication is being administered in the setting of only one clinician (e.g., home care nurse).
- Use pumps with safety features such as dose alerts, dosing/flow rate limits, and pump malfunction alerts; use one type of pump throughout the organization whenever possible.
- Provide staff education with an opportunity to program and connect infusion pumps used at the institution.
- Educate patients and caregivers about when to call, the total dose they are receiving, the length of time the infusion should last, and occasionally checking the remaining drug volume in the pump.
- Report any issues following institutional protocols, and file a voluntary report with FDA.